User Documentation Molecular Usecase
This is the user documentation for the QuCUN material science use case. In the material science use case the quantum computer is used to obtain the ground state energy and derived properties of a molecule consisting of two atoms. It is an example of simulation use cases for quantum computers where the quantum computers can be used to solve an inherently quantum simulation problem that in general would require exponential resources on a convetional computer. This user documentation gives a general introduction of the use case and the methods used here.
Table of Contents
Introduction
An important property of molecules is the dissociation profile, the ground state energy of the molecule over the distance between two atoms in the molecule. From the dissociation profile, properties like the dissociation energy, the equilibrium distance in the molecule and the oscillation frequency of the atoms in the molecule can be obtained.
In this use case, we limit ourselves to the simple case of a molecule consisting of two atoms. For this case, the definition of the distance is clear and the number of molecular orbitals that needs to be involved in the calculation can be small enough to fit on a quantum computer.
The dissociation profile is obtained from the quantum computer using a VQE
(Variational Quantum Eigensolver) with a Unitary Coupled-Cluster Single
Double (UCCSD) ansatz. VQE is a variational approach, which
means that this is a hybrid algorithm where free parameters of the VQE
algorithm are optimized by a classical optimizer during run time. For a more
detailed discussion of the quantum algorithm used in this use case, see the
VQE
section.
The quantum solver is based on the
qiskit
library, which provides a general quantum computing toolkit as well as a
selection of quantum algorithms (like the VQE) and ansätze for the VQE.
The derived properties (equilibrium distance r0, dissociation energy ΔE and oscillation frequency ω) are obtained by pure classical post-processing fitting the VQE dissociation profile to the Morse Potential. For an example for extracting the properties for a Hydrogen atom, see the Dissociation profile section.
As a variational method, the quality of the results of the VQE can strongly depend on the exact parameters chosen for the calculation.
Molecular Dissociation
Dissociation profile for the hydrogen molecule
In this materials use case the VQE algorithm is used to calculate the ground
state energies of a molecule across different interatomic distances on a
quantum computer. The so obtained dissociation profile of the
molecule (here: H2) is compared to the results obtained from the pure
Hartree Fock method and to the conventional full CI calculation (referred to
as "exact" here).
This plot shows a dissociation profile for the hydrogen molecule calculated
in the 6-31g (reduced)
basis, where orbitals [2, 3] have been removed from the active space making
it a minimal basis set to reduce calculation runtime. Similar to the
conventional methods of quantum chemistry, a small basis set leads to a
limited precision of the obtained results.

As one can see from a simple demonstration, the obtained results from the quantum algorithm (here: VQE) show a good agreement with the (exact) full CI calculation. Moreover, its accuracy (here: on an ideal quantum computer) is much higher than of the classical Hartree Fock method, which has been used as the initial ansatz here. Also the true equilibrium distance (vertical dotted line) and the expected dissociation limit (horizontal dotted line) can be described with a sufficient quality, although only a minimal basis set was used for the calculations.
Molecule properties
The obtained VQE dissociation curve allows us to derive important molecule properties, like equilibrium distance r0, dissociation energy ΔE and oscillation frequency ω by pure classical post-processing. The use case extracts these properties by fitting the Morse Potential to the dissociation curve obtained from the VQE (or to the other results obtained from the use case).
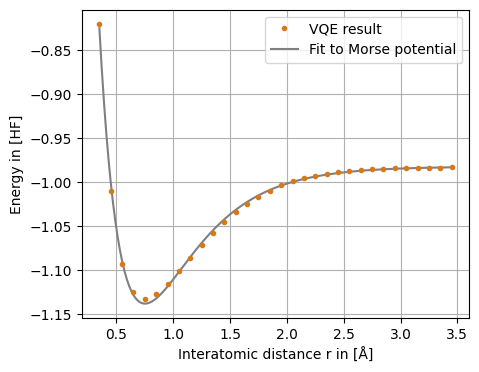
We see that the VQE (Hartree-Fock, exact) results can be fitted quite nicely by the Morse Potential.
The desired molecule properties, like equilibrium distance r0, dissociation energy ΔE and oscillation frequency ω, can be obtained analytically from the Morse Potential fit.
Specifically for the given example, given the limited precision when running measurements with minimal basis sets, the quantum algorithm (on a perfect simulated quantum computer) achieves results of a similar quality as the classical full CI calculation.
Dissociation energy from thermodynamic cycle
There exists an alternative way to compute the dissociation energy of the hydrogen molecule as the heat effect of the reaction: \( H_2 \longrightarrow 2H \). The dissociation energy is then computed as follows: \( \Delta E = 2E(H) - E(H_2) \), where \( E(H) \) is the energy of a single hydrogen atom, while \( E(H_2) \) is the energy of the hydrogen molecule at the minimum of the potential energy curve. Since the hydrogen atom has only one electron, there is no electron correlation, and \( E(H) \) should be calculated with the Hartree-Fock method for all three approaches (VQE, Hartree-Fock, and exact). However, the molecule should be computed with the electronic structure method of choice. It is instructive to compare \( \Delta E \) obtained by this method with the one obtained from the fitted Morse potential.
The reference values for the properties of interest for the Hydrogen molecule from the literature are (see also ref 1):
| Equilibrium distance r0 in [Å] | Dissociation energy ΔE in [Hartree] | Oscillation frequency ω in [1/cm] |
|---|---|---|
| 0.741 | 0.174 | 4404 |
Notes
Please note that for demonstrative purposes a small molecule has been chosen
here, which allows to compare the VQE results to the exact solution.
The VQE calculations shown here ran on a quantum computing simulator,
not on real quantum computer hardware yet. This implies the assumption of an
ideal, i.e. error-free, quantum computer. Whereas real quantum computers are
subject to so called 'noise' (errors) that affects the obtained results.
Results from runs on actual hardware will deviate more strongly from the
exact solution.
Variational Quantum Eigensolver (VQE)
The Variational Quantum Eigensolver (VQE) is hybrid quantum-classical algorithm that can be used to calculate ground state energy of a Hamiltonian (e.g. a molecule) on a quantum computer.
The core principle of the VQE is the variational principle. The ground state is the lowest energy state \(|\psi_0 \rangle \) of all possible states \( \{ |\psi \rangle \}\) of a Fock space, where the energy is the expectation value of a Hamiltonian \(H \). Finding the lowest energy state of a given set of trial states \( \{ |\psi_T \rangle \}\) is a good approximation to finding the ground state and the ground state energy as long as the set of trial states covers the Fock space densely enough that a trial state is close to the true ground state.
The VQE uses the quantum computer to prepare the trial states \( |\psi_T(\theta) \rangle \) as a function of some classical parameters \(\theta \). The expectation value of the Hamiltonian is then measured on the quantum computer as well. The hybrid part of the VQE is a classical optimizer. The classical optimizer tries to minimize the cost function, the expectation value of the Hamiltonian, by varying the classical parameters \(\theta \). Once converged to an optimal set of parameters \(\theta_0\) the approximation of the ground state is given by \[ | \psi_0 \rangle \approx | \psi_T(\theta_0) \rangle \] and the approximation of the ground state energy is given by the final value of the cost function. Besides the ansatz for the trial wave functions, the chosen optimizer can have a large influence on the quality of the VQE results. For a list of the available optimizers see section Implementation Details.
For a detailed discussion of the Variational Quantum Eigensolver, we refer the reader to paper by McClean et al..
Ansatz
As mentioned above, the ansatz for the trial wave functions is an important
choice for the VQE algorithm. We use the Unitary Coupled-Cluster Singles and
Doubles (UCCSD) ansatz for the VQE algorithm. This ansatz is a standard
choice for the VQE algorithm when considering quantum chemistry problems and
is implemented in qiskit. The ansatz state is constructed
starting from a Hartree-Fock state, and then acting on it with some
exponential "cluster" operators that generate excitations to account for
electron correlation. Electron correlation built this way accounts for
electron-electron repulsion better than the average field used by
Hartree-Fock. More precisely, while Hartree-Fock only includes exchange
correlation between electrons (i.e. the correlation due to antisymmetry,
also known as Fermi correlation), the UCCSD ansatz improves on it by
partially accounting for Coulomb correlation.
The wavefunction of the coupled-cluster theory is written as an exponential ansatz: \[ | \psi \rangle = e^T | \Phi_0 \rangle \] where \( | \Phi_0 \rangle \) is a Slater determinant obtained from Hartree-Fock molecular orbitals. The action of the exponentiated cluster operator \( T \) on the state \( |\Phi_0\rangle \) produces a linear combination of Slater determinants that partly account for Coulomb interaction.
The cluster operator has the form \( T = T_1 + T_2 \) , where \( T_1 \) generates single-electron excitations and \( T_2 \) generates two-electron excitations, from which the naming "Singles and Doubles" in the UCCSD acronym stems. The expressions for the excitation operators are \[ T_1 = \sum_{ia} t_{ia} c^{\dagger}_a c_i + h.c. \] \[ T_2 = \frac{1}{4} \sum_{ijab} t_{ijab} c^{\dagger}_a c^{\dagger}_b c_i c_j + h.c. \] where \( t_{ia} \) and \( t_{ijab} \) are real coefficients, \( c^{\dagger} \) and \( c \) are fermionic creation and annihilation operators, and the indices \( i, j \) correspond to occupied molecular orbitals in the original Hartree-Fock state \( | \Phi_0\rangle \), while \( a, b \) correspond to unoccupied states. The \( t_{ia} \) and \( t_{ijab} \) are the free parameters that are optimized by the classical optimizer to find the minimum energy.
While the operators \( T_1 \) and \( T_2 \) generate respectively single and double excitations when acting directly on \( |\Phi_0\rangle \), notice that when exponentiated as \( e^T \) it also generates higher correlations due to the power series expansion of the exponential.
Implementation
In this section we discuss the implementation details of the use cases and the available options that can be chosen by the (expert) user.
The use cases is implemented in Python with the
qiskit
quantum computing framework. The use case will run on the QuCUN platform.
Whether it runs on a noise-less quantum simulator, a noisy quantum simulator
or on actual quantum hardware is determined by the QuCUN platform settings.
When running on an ideal, i.e. error-free quantum computer simulator, the
simulation results can be very accurate, depending on the chosen parameters.
Real quantum computer hardware is subject to environmental noise (leading to
errors) that affects the obtained results negatively.
Chemical drivers and basis choices
The UCCSD ansatz used by the use case needs the Hartree-Fock self consistent
solution of the molecule as an input. This solution can be obtained by
qiskit from a chemical driver. The use case supports two chemical drivers:
PySCF
and Psi4. The default choice for a chemical driver to perform
the Hartee-Fock calculation is PySCF.
The chemical driver takes the input molecule, as well as the
information on the basis set, to define the
electronic structure problem.
In computational chemistry, a basis set is a finite set of functions, usually chosen to be orthogonal, that are used as a basis to approximate electronic wavefunctions. As the number of basis functions in the set approaches infinity, the accuracy of the approximation of the electronic wavefunction calculated using the basis set improves at the price of a higher computational cost.
Basis sets are used for example to calculate the molecular orbitals that serve as the Hartree-Fock starting point for the construction of the UCCSD ansatz, but also to construct the ansatz from the initial Hartree-Fock state. The choice of basis set is influenced by the tradeoff between computational resources available and accuracy of the desired result. A basis set is called minimal if for each atom in the molecule it contains only enough orbitals to contain the electrons in the neutral atom. Thus for the hydrogen atom only a single 1s orbital is needed, while for a carbon atom, 1s, 2s and three 2p orbitals are needed. The basis sets available are:
-
STO3G : Minimal basis set in which each basis function is expressed as a linear combination of 3 primitive Gaussian functions. Both core and valence orbitals are represented in this way. Allows for fastest but least accurate calculations.To keep runtimes of simple test runs low, this is the default option. -
6-31G : Split-valence basis set, i.e. the core orbitals and the valence orbitals are represented differently. Here, the core orbitals are represented as a linear combination of six Gaussian functions, while the valence orbitals are represented with two basis functions, expressed as linear combinations of respectively three and one primitive Gaussian function. Gives more accurate results but has longer run times. -
MINAO (MINimal Atomic Orbital) : Tradeoff between accuracy and computational cost.
Classical Optimizers
The cost function that we want to minimize is the expectation value of the fermionic Hamiltonian of interest, evaluated in the UCCSD ansatz state with a certain set of parameter values. The parameter values are then optimized by a classical optimizer. The classical optimizers available are:
- L-BFGS-B (Limited-memory BFGS Bound): It is a limited-memory approach, meaning that doesn't explicitly store the Hessian matrix, which can be computationally expensive for large-scale problems. Instead, it maintains an approximation of the inverse Hessian matrix using limited memory. This makes it suitable for optimization problems with a large number of variables. As a gradient-based optimizer L-BFGS-B gives good results for noise-less quantum simulator but is sensitive to noise in the cost function due to noise on the quantum computer.
-
COBYLA
(Constrained Optimization BY Linear Approximations): COBYLA belongs to the
class of direct search or derivative-free optimization methods. This means
that it does not rely on gradient information (first or second
derivatives) of the objective function or constraints. Instead, it uses a
pattern of linear approximations to estimate the direction of descent or
ascent in the objective function space while respecting the constraints.
As such, COBYLA is particularly useful when the objective function or
constraint functions are noisy, non-smooth, or difficult to compute
derivatives for.
For this reason, we use COBYLA as the default optimizer as it is suitable for actual quantum computing hardware, where we encounter noise in the quantum computer. - SLSQP (Sequential Least Squares Quadratic Programming): SLSQP utilizes gradient information (first derivatives) of the objective function and constraints, if available. This makes it an efficient choice for optimization problems with smooth functions where gradient information can be computed or approximated. SLSQP employs a sequential quadratic programming approach, which involves approximating the objective function and constraints with quadratic models and then iteratively solving subproblems to optimize these models within the constraint boundaries. This process continues until convergence to the optimal solution.
- SPSA (Simultaneous Perturbation Stochastic Approximation): SPSA is a derivative-free optimization method that falls under the umbrella of stochastic approximation techniques. SPSA does not require gradient information (first or second derivatives) of the objective function. Instead, it approximates the gradient by perturbing the decision variables simultaneously and using finite differences to estimate the change in the objective function.
- NFT (Nakanishi-Fujii-Todo): It is a sequential minimal optimization method specifically designed for hybrid quantum-classical algorithms.
- CG (Conjugate Gradient): An iterative numerical optimization algorithm used for solving unconstrained optimization problems, particularly those that involve quadratic objective functions. CG is an iterative method, which means it refines an initial guess for the solution in a series of steps. It does not require the explicit calculation or storage of the Hessian matrix, making it suitable for large-scale problems.
In the official SciPy documentation additional information on some of these optimizers is available.
Qubit Mapper
The qubit mapper translates the fermionic degrees of freedom in the original quantum chemisty problem into spin degrees of freedom that can be represented on a quantum computer. Several different mappings are possible, in qiskit we have two options
-
ParityMapper- Encodes the parity of all teh fermionic orbitals up to an orbitaljin qubitj. When providing the overall number of fermionic particles in the system, the qiskit ParityMapper can reduce the number of qubits by1.We use this mapping as the default. -
JordanWigner- The JordanWigner mapping encodes the occupation of an fermionic orbitaljin qubitj.
For a more detailed discussion of mappings in the context of quantum simulation see for example this paper by Seeley et al.
Inactive orbitals
The number of required qubits in the system increases linearly with the
number of molecular orbitals in the problem. When the number of qubits
becomes too large for the available hardware the number of qubits required
can be reduced by reducing the active space. Orbitals that are always
occupied and orbitals that are always unoccupied are removed from the
problem in a transformation. Removing orbitals from the active space speeds
up calculations, but depending on the molecule being simulated can impact
the accuracy of the results. Core orbitals are always removed from the
active space automatically. The user can additionally remove unoccupied
orbitals with the inactive_orbitals orbitals option that lists
the orbitals that should be removed as unoccupied by the transformation.
Qiskit does not perform a check when removing the unoccupied orbitals and
this option should only be used by expert users. For details on the
mathematical transformation involved
see.
Calculated results
For better comparison the use case calculates three results for the dissociation profile and the post-processing:
- The
VQEresult -
The
Hartee-Fockresult taken directly from the chemical driver -
The
exact> solution. The exact solution corresponds to solving for the ground state of the effective Hamiltonian obtained from the chemical driver by exact diagonalisation. In chemical terms it would be equivalent to Full CI, but it is not a direct diagonalisation of the original fermionic Hamiltonian.
Ressources
[1]
Experimental data for H2
, https://cccbdb.nist.gov/exp2x.asp?casno=1333740
[2] Scientific
paper
on the improved theoretical ground state energy of the hydrogen molecule
[3] Scientific
paper
on the potential energy curves